Delivering Therapy Where it Matters
Developing Innovative Combination Therapies for Targeted Treatment of Difficult-to-Access Cancers
Developing Innovative Combination Therapies for Targeted Treatment of Difficult-to-Access Cancers
The Phase III TIGeR-PaC clinical trial is an ongoing randomized multi-center study using RenovoRx’s innovative therapy platform, Trans-Arterial Micro-Perfusion (TAMP™), to evaluate RenovoRx’s first product candidate, RenovoGem™. The study is evaluating trans-arterial delivery, a form of intra-arterial administration, of an FDA-approved chemotherapy, gemcitabine, to treat LAPC following stereotactic body radiation therapy (SBRT). The study is comparing treatment of gemcitabine with TAMP versus systemic IV administration of gemcitabine and nab-paclitaxel.
RenovoRx’s proprietary Trans-Arterial Micro-Perfusion (TAMP) therapy platform is designed to ensure precise therapeutic delivery to directly target the tumor while potentially minimizing a therapy’s toxicities versus systemic (intravenous (IV) therapy). RenovoRx’s unique approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy. Our Phase III lead product candidate, RenovoGem, a novel oncology drug-device combination product, is being investigated under a US IND that is regulated by FDA 21 CFR 312 pathway. RenovoGem is currently being evaluated for the treatment of locally advanced pancreatic cancer (LAPC) by the Center for Drug Evaluation and Research (the drug division of FDA.)
RenovoGem will also be evaluated as a potential therapy in bile duct cancer, with a Phase III clinical trial expected to begin in late 2023 with other potential pipeline indication opportunities to follow including non-small cell lung cancer, uterine tumors, glioblastoma, and sarcoma. RenovoGem received FDA Orphan Drug Designation for pancreatic cancer and bile duct cancer which provides 7 years of market exclusivity upon NDA approval.
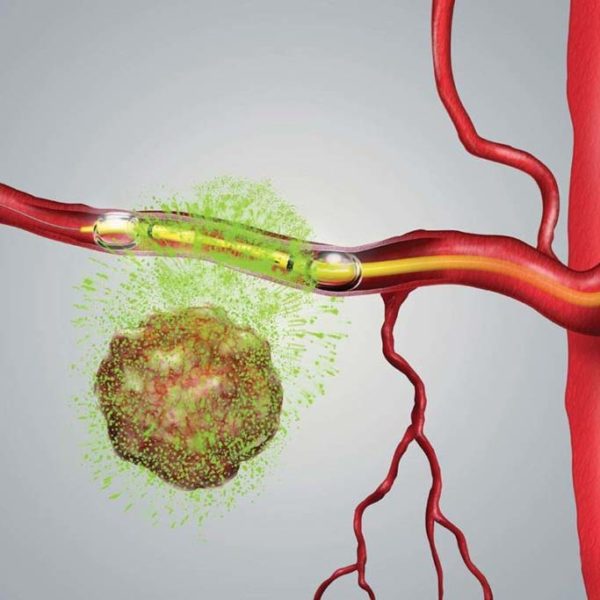
RenovoRx is a clinical-stage biopharmaceutical company developing proprietary targeted combination therapies for high unmet medical need with a goal to improve therapeutic outcomes for cancer patients undergoing treatment. We are committed to developing transformative therapies for improved quality of life and extended life in cancer patients.