Trans-Arterial Micro-perfusion (TAMP™) is an Innovative Therapy Platform Designed for Targeted Delivery of Existing and Novel Oncology Agents to Tumors
The TAMP therapy platform is designed to ensure precise therapeutic delivery to directly target the tumor while potentially minimizing a therapy’s toxicities versus systemic (intravenous (IV) therapy). RenovoRx’s unique approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy. Our Phase III lead product candidate, RenovoGemTM, a novel oncology drug-device combination product, is being investigated under a US IND that is regulated by FDA 21 CFR 312 pathway. RenovoGem is currently being evaluated in the Phase III TIGeR-PaC clinical trial for the treatment of locally advanced pancreatic cancer (LAPC) by the Center for Drug Evaluation and Research (the drug division of FDA.)
Learn more about TAMP by watching this animation:
Localized and Targeted Therapy
The therapeutic approach of TAMP is specifically designed for the localized and targeted delivery of chemotherapy via the peripheral vascular system. Our patented delivery system is inserted into an artery that runs adjacent to the tumor via a small incision made in the patient’s leg. The delivery system’s double balloon design enables the physician to isolate sections of the blood vessel through the adjustment of the distance between the balloons, thereby excluding any branching blood vessel in order to create the pressure head needed to push chemotherapy across the blood vessel wall and bathe the tumor in chemotherapy.
Standard of care for patients diagnosed with most forms of cancer is intravenous systemic chemotherapy, which typically has significant side effects and can be limited in its effectiveness based on cancer type. For example, liver cancer tumors are highly vascularized and typically have large tumor feeders or blood vessels connected to the tumor, making them ideal candidates for systemic chemotherapy because medicine is able to gain direct access to the tumor. In contrast, pancreatic cancer tumors lack visible tumor feeder blood vessels, which means the chemotherapy circulates through the body, without a significant amount of medicine reaching the tumor.
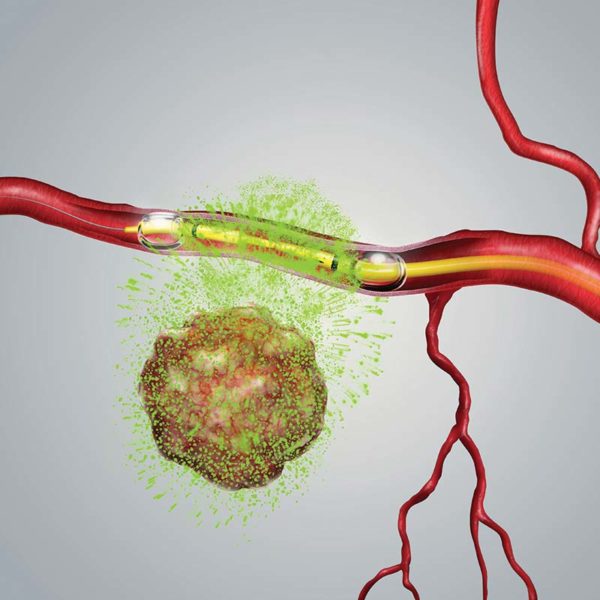

Targeted Delivery In Action
The first of two interim analyses was completed in March 2023, and the DMC recommended a continuation of the study. The study is prespecified to provide a primary endpoint of a 6-month OS benefit and secondary endpoints including reduced side effects versus standard of care.
Caution: Federal (USA) law restricts this system to sale by or on the order of a physician.
The RenovoRx delivery system (“RenovoCath”) is intended for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system. The RenovoRx delivery system is also indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion. Prior to use, please refer to the RenovoRx IFU for complete product indications, warnings, precautions, contraindications, potential adverse effects and detailed instructions for use.
