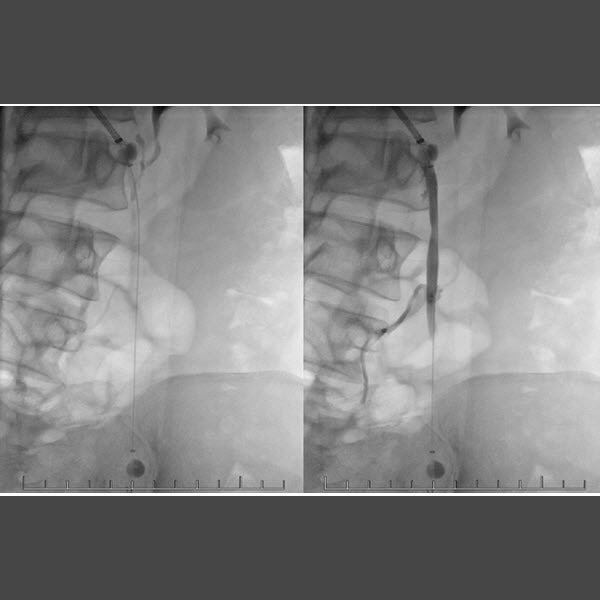
RenovoRx today announced the first in-human use of the RenovoCath™ catheter. RenovoRx is a leading developer of innovative solutions for targeted delivery of fluids, including therapeutic agents, to selected sites in the vascular system. The patient underwent localized large vein sclerotherapy for pelvic congestion syndrome at El Camino Hospital by Dr. Fabio Komlos, an interventional radiologist at El Camino Hospital, in consultation with Dr. Chuck Martin, an interventional radiologist at the Cleveland Clinic.
“We identified a woman suffering from pelvic congestion syndrome who would potentially benefit from targeted delivery of a sclerosing agent using the RenovoCath to shut down diseased veins. The treatment was successful and the patient’s chronic pelvic pain has been reduced as a result,” said Dr. Komlos. “The potential for interventionalists to use an adjustable infusion catheter such as the RenovoCath for direct delivery of therapy to regions of the peripheral vascular system may have significant impact on the care we provide to patients.”
The RenovoCath catheter is specifically designed for the isolation of blood flow and delivery of fluids, including diagnostic material and therapeutic agents, into selected sites in the peripheral vascular system. The innovative design allows clinicians to deliver diagnostic and therapeutic agents to the visceral and peripheral vascular system to provide precise, controlled infusion of fluids to targeted regions, which may potentially increase effectiveness of treatment and reduce side effects. The RenovoCath received FDA clearance in late 2014.
“This is a major milestone for RenovoRx,” said Shaun Bagai, Chief Executive Officer. “We are delighted that the RenovoCath performed successfully in our first patient. As we continue our market introduction, we look forward to reporting on the successful use of the RenovoCath in helping physicians deliver therapeutics in a targeted manner.”