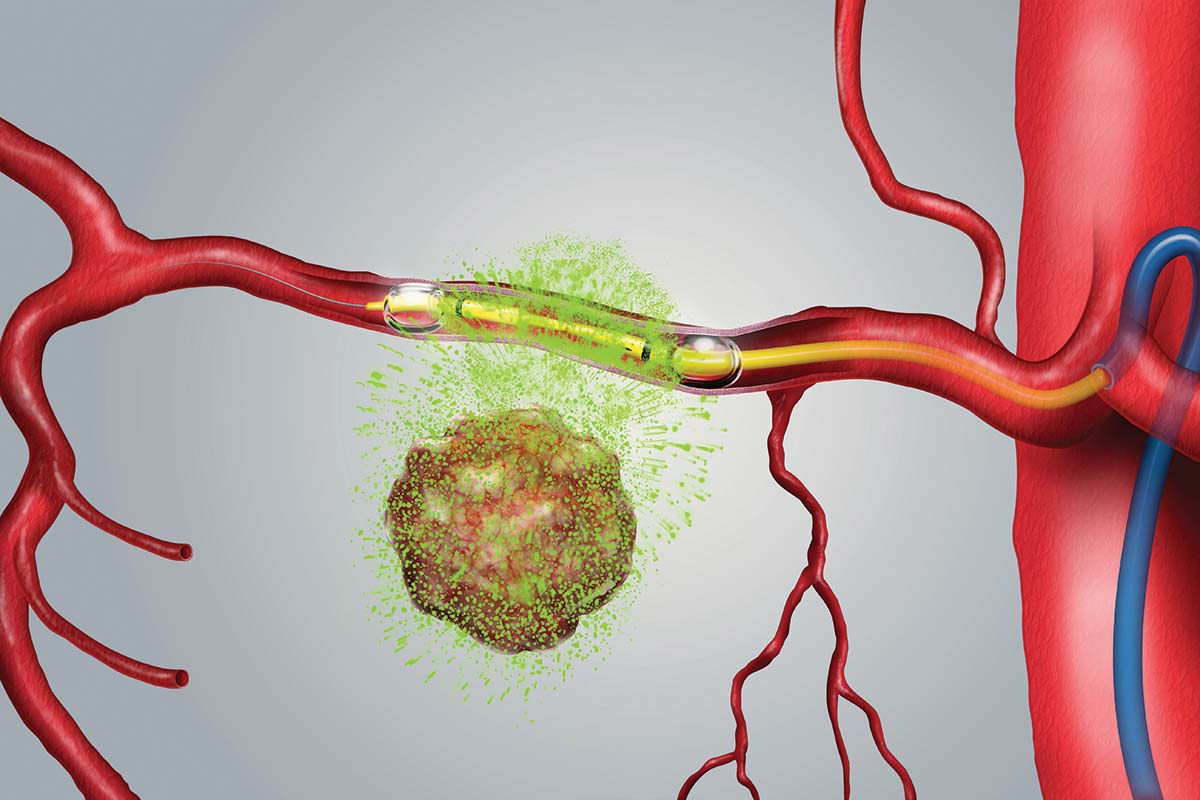
Los Altos, CA, February 22, 2022 – RenovoRx, Inc. (“RenovoRx” or the “Company”) (Nasdaq: RNXT), a biopharmaceutical company and innovator in targeted cancer therapy, today announced enrollment of its first patient at one of its newest clinical sites, Columbia University New York-Presbyterian Hospital Irving Medical Center (“Columbia University”), in its ongoing TIGeR-PaC Phase 3 clinical trial. The study is evaluating the Company’s therapy platform RenovoTAMP® (RenovoRx Trans-Arterial Micro-Perfusion), as a potential treatment option for locally advanced pancreatic cancer (“LAPC”) that may extend life while improving quality of life by reducing the often debilitating side-effects associated with systemic chemotherapy delivered intra-venously.
Columbia University is the most recent trial site to join the Phase 3 study which continues enrolling patients at participating clinical sites across the U.S.
“Cancer of the pancreas is aggressive and difficult to detect and treat.” said Principal Investigator Susan Bates, M.D., Professor of Medicine at Columbia University Irving Medical Center.
Dr. Bates added, “Treatment options have been limited to systemic chemotherapy for most patients. Local perfusion allows us to deliver much higher concentrations of an effective agent to the tumor. In the TIGeR-PaC trial, the tumor is temporarily isolated and saturated with gemcitabine, a well validated chemotherapeutic agent. In comparison to systemic chemotherapy treatment, RenovoRx has shown in its Phase 1/2 clinical trials that its therapy platform delivers a higher concentration of chemotherapy directly to the tumor with reduced patient side effects. This trial will offer a proof-of-principle that could establish direct delivery of chemotherapy as a strategy for treating pancreatic cancer in various settings.”
Dr. Ramtin Agah, RenovoRx’s Founder and Chief Medical Officer commented, “Dr. Bates and her Columbia University team share our deep commitment to helping pancreatic cancer patients. Enrolling our first patient at this renowned institution is an important milestone for the TIGeR-PaC clinical trial. This also gives more LAPC patients in New York, New Jersey and Connecticut an opportunity to participate in this study.”
To learn more about RenovoRx’s ongoing clinical trials, please visit the Clinical Trials page of our website.
About the Phase 3 TIGeR-PaC Clinical Trial
The TIGeR-PaC clinical trial is a randomized multi-center study using the RenovoTAMP platform to evaluate RenovoRx’s first product candidate, RenovoGem™ to treat unresectable LAPC through the intra-arterial delivery of gemcitabine. TIGeR-PaC is currently enrolling locally advanced, unresectable pancreatic cancer patients. To learn more about the study and the participating clinical trial sites, visit https://renovorx.com/clinical-trial/.
About RenovoRx, Inc.
RenovoRx is a clinical-stage biopharmaceutical company focused on fighting cancer through the localized treatment of difficult to treat tumors via its proprietary RenovoRx Trans-Arterial Micro-Perfusion (RenovoTAMPTM) therapy platform. RenovoTAMP utilizes approved chemotherapeutics with validated mechanisms of action and well-established safety and side effect profiles, with the goal of increasing their efficacy, improving their safety, and widening their therapeutic window. RenovoRx’s lead product candidate, RenovoGemTM, is a combination of gemcitabine and our patented delivery system, RenovoCath®, and is regulated by the FDA as a novel oncology drug product to treat unresectable locally advanced pancreatic cancer (LAPC). RenovoGem is currently being studied in the Phase 3 TIGeR-PaC trial for the treatment of LAPC.
RenovoRx’s patent portfolio includes seven U.S. patents for its technology. RenovoRx has been granted Orphan Drug Designation for intra-arterial delivery of gemcitabine for the treatment of both pancreatic cancer and bile duct cancer.
RenovoRx won the Drug Delivery Technology category of the Fierce Innovation Awards – Life Sciences Edition 2020 for its RenovoTAMP technology.
Learn more by visiting the RenovoRx website or following us on Facebook, LinkedIn and Twitter.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934, including but not limited to statements regarding our Phase 1 (RR1) and Observational Registry (RR2) studies, statements regarding the potential of RenovoTAMPTM, RenovoCath® or RenovoGemTM or regarding our ongoing TIGeR-PaC Phase 3 clinical trial in LAPC, and statements regarding the potential for our product candidates to treat or provide clinically meaningful outcomes for certain medical conditions or diseases. Statements that are not purely historical are forward-looking statements. The forward-looking statements contained herein are based upon our current expectations and beliefs regarding future events, many of which, by their nature, are inherently uncertain, outside of our control and involve assumptions that may never materialize or may prove to be incorrect. These may include estimates, projections and statements relating to our research and development plans, clinical trials, therapy platform, business plans, objectives and expected operating results, which are based on current expectations and assumptions that are subject to known and unknown risks and uncertainties that may cause actual results to differ materially from those expressed or implied by these forward-looking statements. These statements may be identified using words such as “may,” “expects,” “plans,” “aims,” “anticipates,” “believes,” “forecasts,” “estimates,” “intends,” and “potential,” or the negative of these terms or other comparable terminology regarding RenovoRx’s expectations strategy, plans or intentions, although not all forward-looking statements contain these words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, that could cause actual events to differ materially from those projected or indicated by such statements, including, among other things: the timing of the initiation, progress and potential results of our preclinical studies, clinical trials and our research programs; our ability to use and expand our therapy platform to build a pipeline of product candidates; our ability to advance product candidates into, and successfully complete, clinical trials; the timing or likelihood of regulatory filings and approvals; our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; the commercialization potential of our product candidates, if approved; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; future strategic arrangements and/or collaborations and the potential benefits of such arrangements; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing and our ability to obtain additional capital; the sufficiency of our existing cash and cash equivalents to fund our future operating expenses and capital expenditure requirements; our ability to retain the continued service of our key personnel and to identify, hire and retain additional qualified personnel; the implementation of our strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights, including our therapy platform, product candidates and research programs; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; the pricing, coverage and reimbursement of our product candidates, if approved; developments relating to our competitors and our industry, including competing product candidates and therapies; negative impacts of the COVID-19 pandemic on our operations; and other risks.
Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that we file from time to time with the Securities and Exchange Commission.
Forward-looking statements included herein are made as of the date hereof, and RenovoRx does not undertake any obligation to update publicly such forward-looking statements to reflect subsequent events or circumstances, except as required by law.
Company Contact:
RenovoRx, Inc.
Shaun R. Bagai, CEO
Christopher J. Lehman, CFO
Investor Contact:
KCSA Strategic Communications
Valter Pinto or Jack Perkins
T: 212-896-1254
RenovoRx@kcsa.com
Media Contact:
Knight Marketing Communications, Ltd.
Kevin Knight
T: 206-451-4823
kknightpr@gmail.com