Company’s RenovoCath System Has Received Orphan Drug Designation from the FDA for its Innovative Approach.
LOS ALTOS, Calif. – May 23, 2018 – RenovoRx, Inc., a medical technology company developing an innovative catheter-based approach to treating pancreatic cancer, today announced a $10 million financing round of which the company closed $7 million in an initial tranche. The round is led by Boston Scientific and joined by new investors including btov Partners and existing investors Astia Angels, the Angels’ Forum, the Halo Fund III, L.P., Golden Seeds, and Acorn Campus Taiwan.
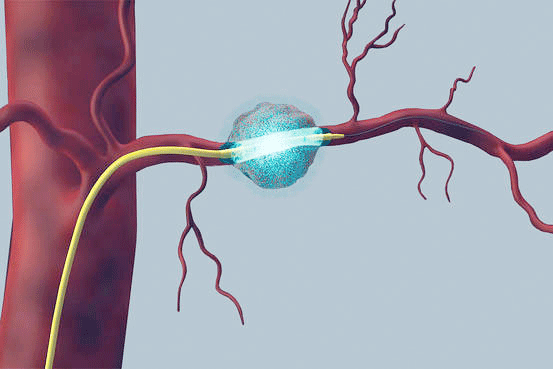
RenovoRx is developing a new approach to treating solid tumors – the RenovoCath™ System, a novel drug-device combination designed to deliver chemotherapy directly to tumors. The financing will be used to support ongoing product development and clinical trials of RenovoCath for the treatment of patients with locally advanced pancreatic cancer.
Pancreatic cancer is one of the deadliest types of cancer. Despite several FDA-approved treatments, it is projected to become the second-leading cause of cancer-related death in the United States around 2020 [1], in part due to challenges in diagnosing and treating the disease. Pancreatic cancer is often not detected until it is advanced and considered inoperable. Standard treatments are often ineffective because they cannot be delivered directly to the tumor and are unable to penetrate the tumor tissue.
Based upon early studies of people with locally advanced pancreatic cancer and the potential to address a significant unmet need, the FDA recently granted RenovoCath an Orphan Drug Designation and approved an Investigational New Drug (IND) application for the company to advance directly to a pivotal Phase III clinical study.
“There are significant challenges in treating pancreatic cancer and our aim is to overcome these barriers by using a catheter-based, targeted approach to delivering chemotherapy. This funding, along with our FDA Orphan Drug Designation and approval of our IND are three important milestones in bringing this new therapy option to patients,” said Shaun R. Bagai, CEO of RenovoRx. “We are excited to begin enrollment in our pivotal Phase III trial of the RenovoCath System and advance this technology in an area of significant patient need.”
RenovoCath is a dual-balloon infusion catheter that reaches pancreatic tumors using a proprietary, catheter-based approach to deliver chemotherapy directly to the tumor inside the pancreas, without the need to identify blood vessels locally at the treatment site. An early feasibility study using this targeted approach suggested an improved survival benefit for people with advanced pancreatic cancer compared to historical controls.
Enrollment has begun in a randomized, Phase III study designed to compare intra-arterial chemotherapy via RenovoCath to systemic chemotherapy in approximately 300 patients with locally advanced pancreatic cancer at up to 20 sites across the United States. The primary endpoint is overall survival (OS) with secondary endpoints including safety and quality of life.
More information can be found at clinicaltrials.gov.
[1] Source: Lola Rahib et al. “Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States.” Cancer Research. Published Online First May 19, 2014.